SSI Institutional Review Board
What We Do
In accordance with the “Research involving Human Subject: Guidelines for IRBs” released by the Bioethics Advisory Committee (BAC) in November 2004, SSI-IRB was formalised in 2012 to safeguard the rights, welfare and safety of human research subjects involved in SSI’s research programmes.
SSI Institutional Review Board (IRB)
The SSI-IRB is comprised of a Chairperson and 9 members from Sport Singapore (SportSG), as well as external agencies/institutions involved in Sport Science, for a 2-year term. The list of the current SSI-IRB members (commencing October 2021 to 30 September 2023) is shown below:
Chairman
Dr Jason Chia
Head & Senior Consultant
Sports Medicine & Surgery Clinic
Tan Tock Seng Hospital
Members
Prof. Michael Chia
Dean
Faculty Affairs
National Institute of Education
Dr Ivy Lim
Director & Consultant
Sport & Exercise Medicine
Changi General Hospital
A/Prof. Chow Jia Yi
Associate Dean
Programme & Student Development
National Institute of Education
Ms Tan Bee Lian
Director
Sports
Singapore Sports School
Mr Matthew James Wylde
Head
Sport Science
National Youth Sports Institute
Mr Lau Kok Keng
Head
Intellectual Property, Sports & Gaming
Rajah & Tann Singapore LLP
Dr Ong Joo Haw
Consultant
Sport Medicine Centre
Khoo Tech Puat Hospital
Dr Alex Ong
Assistant Director / Principal Lecturer
Capability & Industry / Industry
Republic Polytechnic
Dr Marcus Lee
Head
Sport Science and Medicine Centre
Singapore Sport Institute
Quorum for IRB Meeting
At least 5 Members present
-
1 Chairman
-
1 External Layperson
Contact Us
Please contact us for any queries: SPORT_SSI_Research@sport.gov.sg
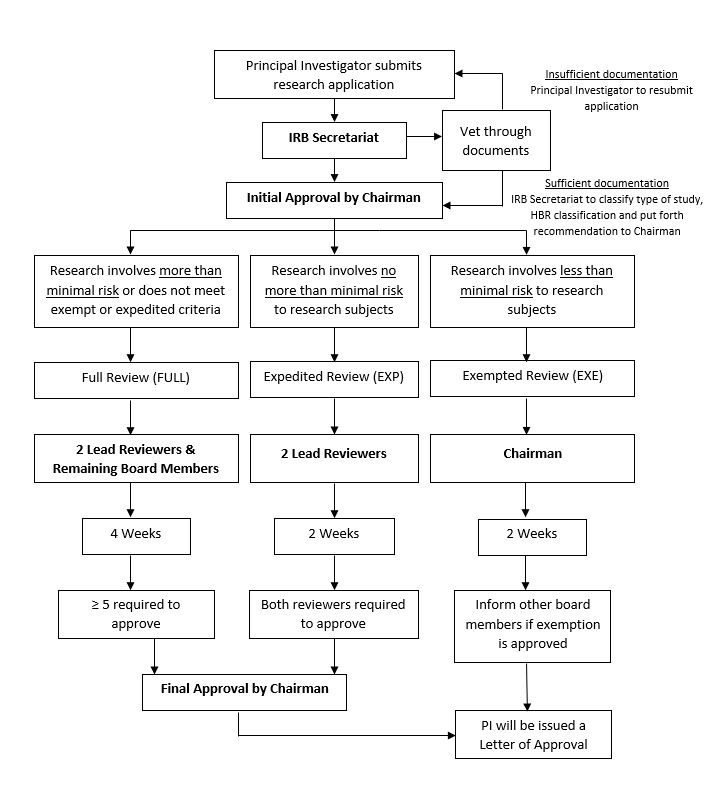
Review Process and Timeline
Application Process
The flowchart below shows the typical process and timeline involved for each application submitted to SSI-IRB. Do note that delays to approval can occur. Some of the common examples causing delay include:
-
Incomplete submissions (Missing necessary documents, forms etc.)
-
Clarifications and queries from the IRB to be addressed
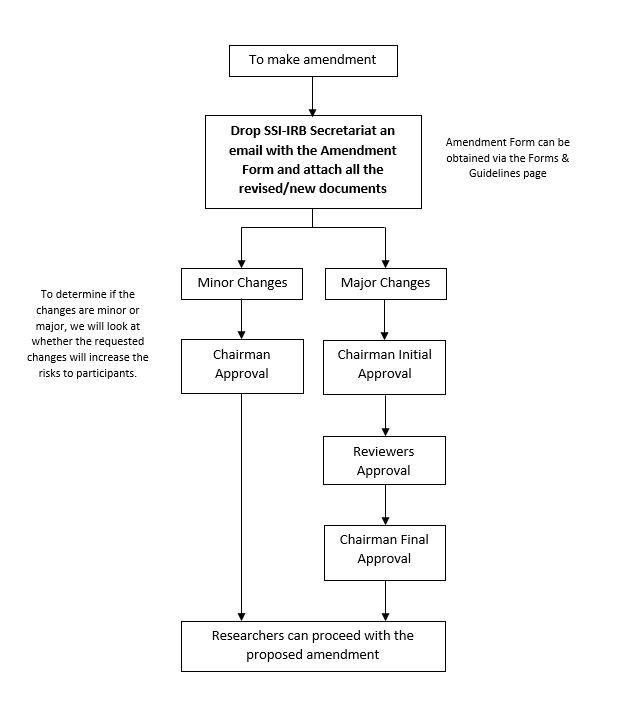
Amendment Process
Researcher should be requesting for amendment when there is a change in:
-
Protocol (design, methodology, procedures, etc.)*
-
Number of participants and/or selection criteria
-
Recruitment materials (flyers, emails, compensation, etc.)*
-
Study materials (surveys, questionnaires, etc.)*
-
Consent form*
-
Research personnel
-
Timeline*
Please ensure that all revised/new documents are submitted to SSI-IRB with the changes highlighted.
Forms and Guidelines
Study pre-approval
|
Document Name |
Version |
Link |
|---|---|---|
|
1. SSI-IRB Application Form |
28 July 2021 |
|
|
2. Specific Research |
||
|
Participant Information Sheet |
05 August 2021 |
|
|
Informed Consent |
12 August 2021 |
|
|
Informed Consent [For Participant Under 21] |
12 August 2021 |
|
|
3. General Research |
||
|
Participant Information sheet, Informed consent, Informed consent [For Participant Under 21] |
12 August 2021 |
|
|
4. Withdrawal / Dismissal Form |
12 August 2021 |
|
|
5. ResearchCollaboration Agreement (RCA) |
August 2020 |
|
|
6. Checklist |
||
|
Checklist HRBA (non-tissue) |
January 2021 |
Download |
Study post-approval
|
Document Name |
Version |
Link |
|---|---|---|
|
1. Amendment Form |
January 2020 |
|
|
2. Incident Reporting |
||
|
SOC Reporting Form |
24 March 2020 |
|
|
SAE Reporting Form |
24 March 2020 |
Guidelines
|
Document Name |
Version |
Link |
|---|---|---|
|
1. IRB Guidelines 01 - Expedited Review |
1 January 2020 |
|
|
2. IRB Guidelines 02 - Exemption from Review |
1 January 2020 |
|
|
3. IRB Guidelines 03 - Human Subjects |
28 April 2021 |
|
|
4. IRB Guidelines 04 - Human Biological Research and Sample |
28 April 2021 |
|
|
5. IRB Guidelines 05 - Requirement of Consent |
28 April 2021 |
|
|
6. IRB Guidelines 06 - Data Management Policy |
1 April 2020 |
|
|
7. SSI-IRB Incident Reporting Guide |
17 April 2020 |
|
|
8. Research Monitoring Guide |
15 January 2021 |
For more information on MOH Guidelines on HBRA, please click here.
Sharing Session
|
Document Name |
Version |
Link |
|---|---|---|
|
1. SSI-IRB Sharing #1 |
24 April 2020 |
|
|
2. SSI-IRB Sharing #2 |
14 April 2020 |
|
|
3. SSI-IRB Sharing #3 |
18 June 2020 |
Research Monitoring
The Principal Investigator (PI) is responsible to provide a status update to the SSI-IRB Secretariat every 6 months (the last week of January and July each year). SSI-IRB Secretariat will provide email reminders when the reporting date is due. Click here for more information on Periodic Update of Research.
Contact Us
Please contact us for any queries: sport_ssi_research@sport.gov.sg

